MAR-T Cell Technology
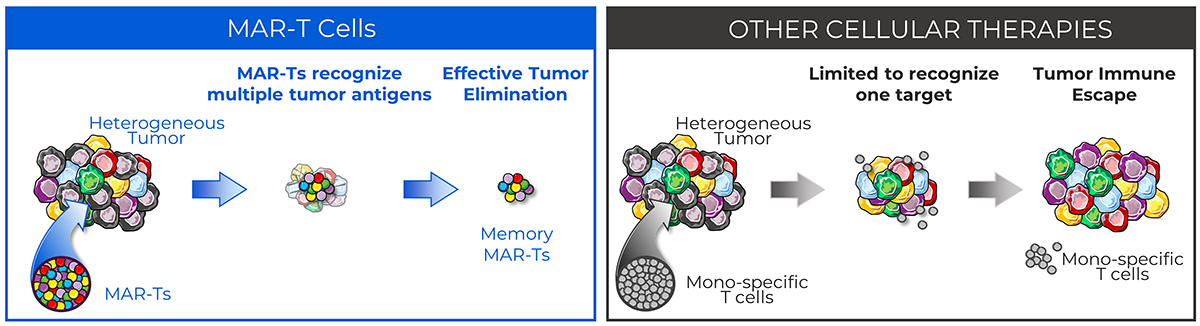
Leveraging millions of years of immunologic evolution, Marker’s unique Multi-Antigen Recognizing T cell (MAR-T cell) technology utilizes a non-genetically modified cell therapy approach that selectively expands natural tumor-specific T cells from a patient’s blood that are capable of recognizing a broad range of tumor antigens. Unlike other cell therapies, MAR-T cells allow the recognition of hundreds of different portions, known as epitopes, within up to six tumor-specific antigens, thereby reducing the possibility of tumor escape for an effective tumor cell killing. Data from our clinical trials have shown that MAR-T cells are well-tolerated, with no ICANS, and have potential clinical benefits such as a broad, potent, and durable anti-tumor response.
