MultiTAA Tabs
Targets Multiple
Tumor Antigens
Marker’s unique MultiTAA technology differs significantly from today’s leading cell therapies, which are genetically engineered to recognize a single portion (or epitope) of a single tumor-associated antigen. MultiTAA-specific T cells recognize up to six antigens for a potent, durable anti-tumor response.
Broad, Durable
Anti-Tumor Effect
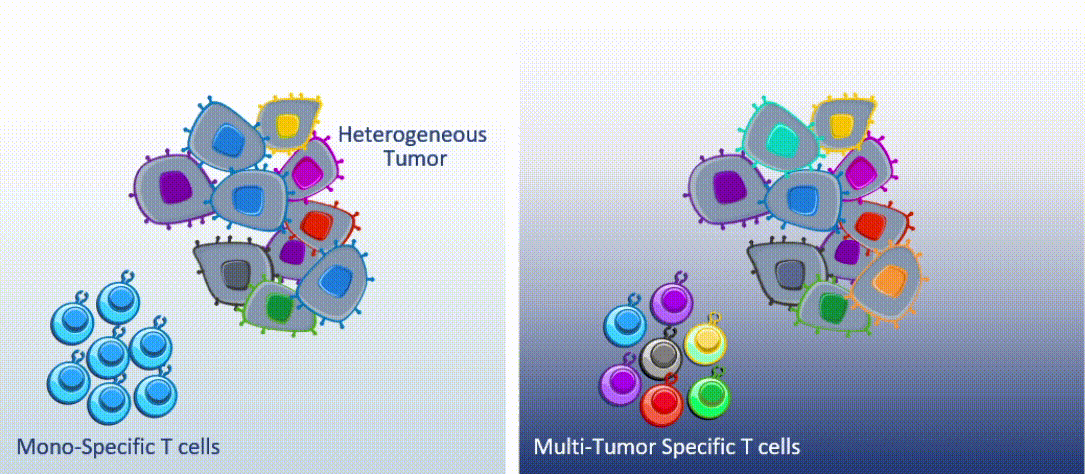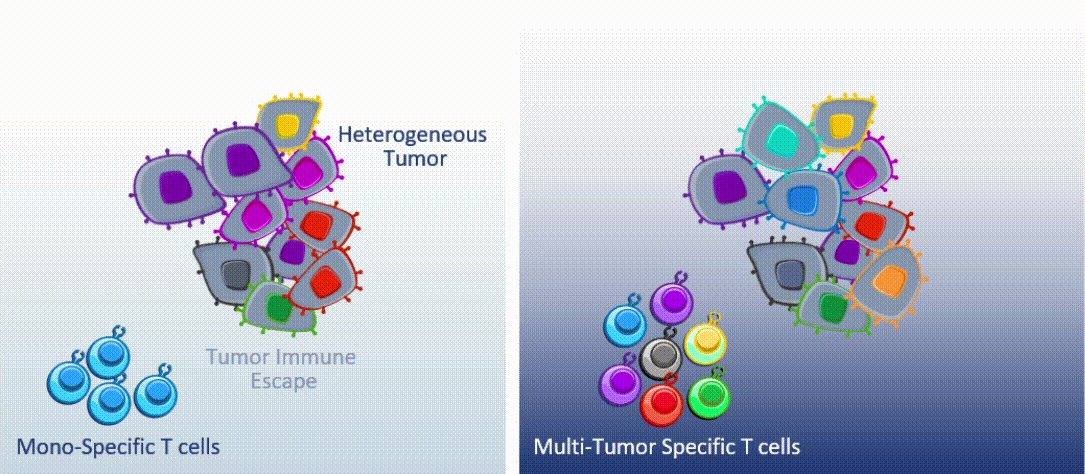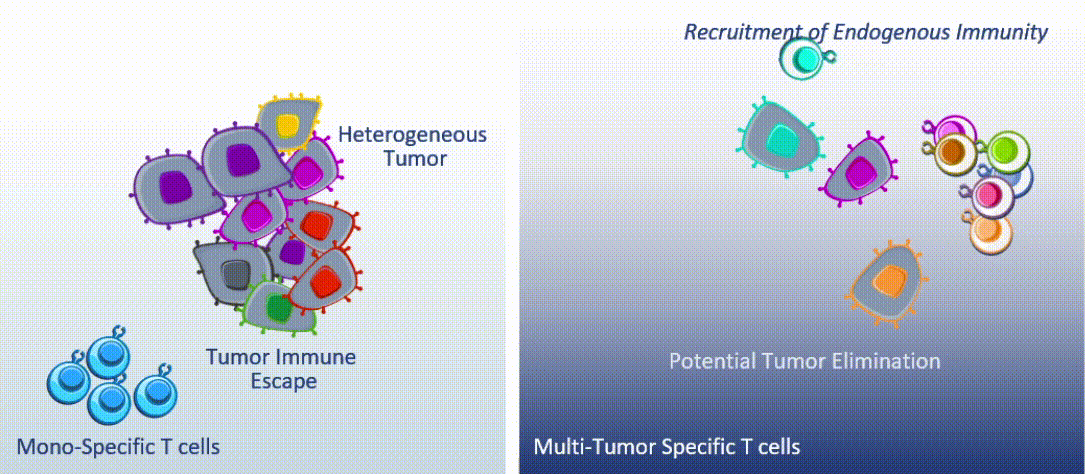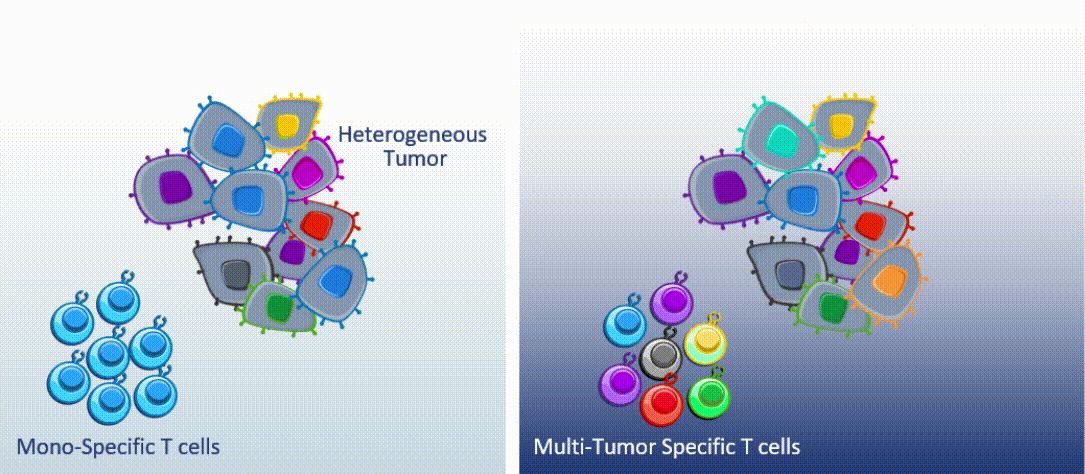
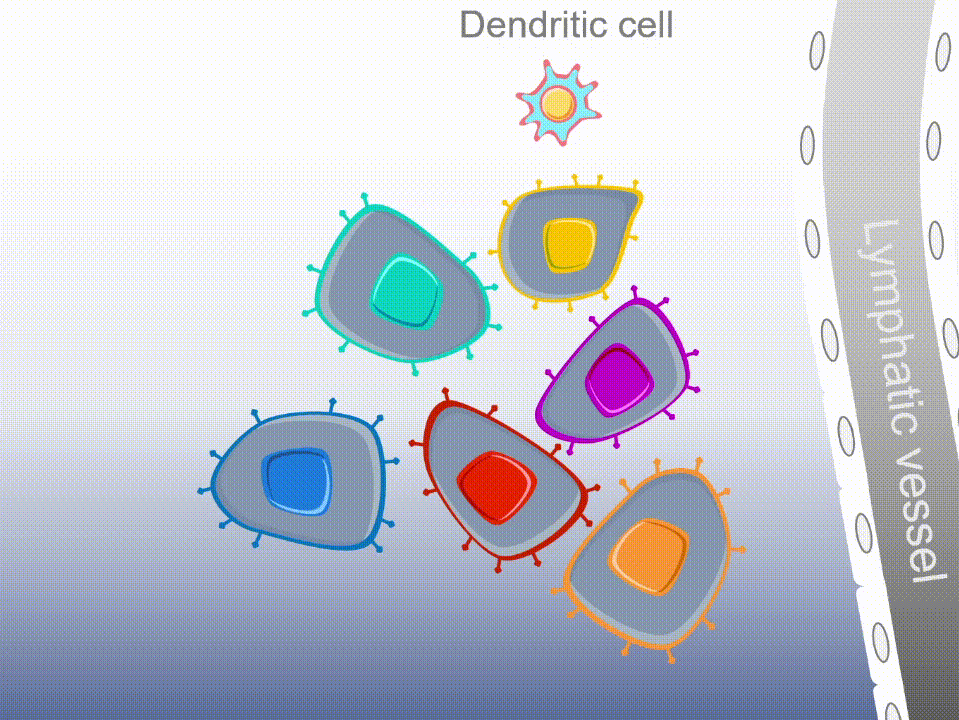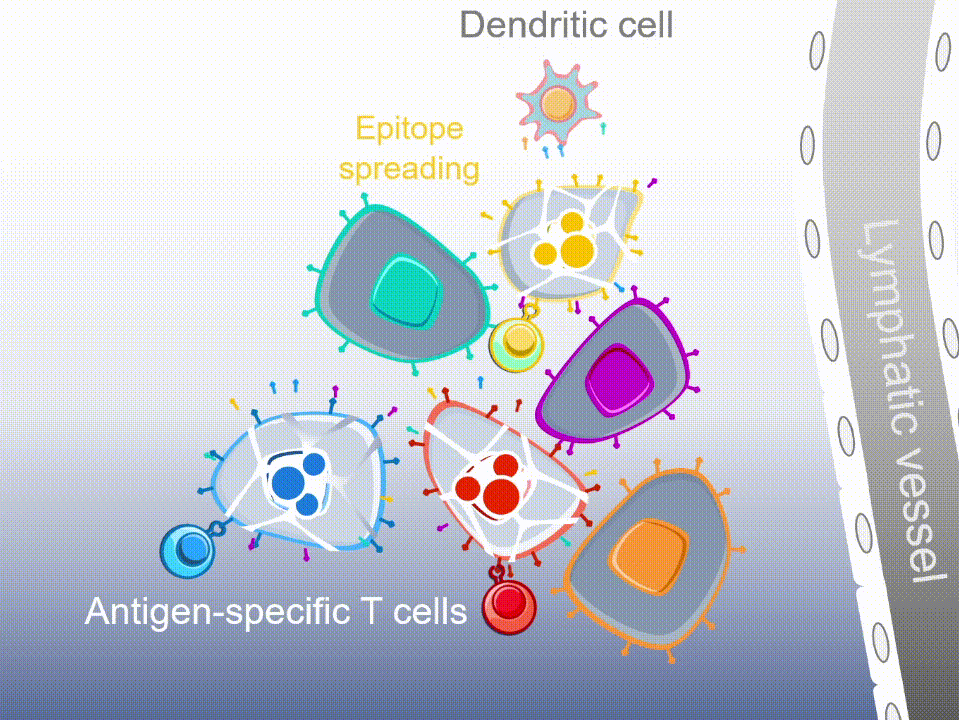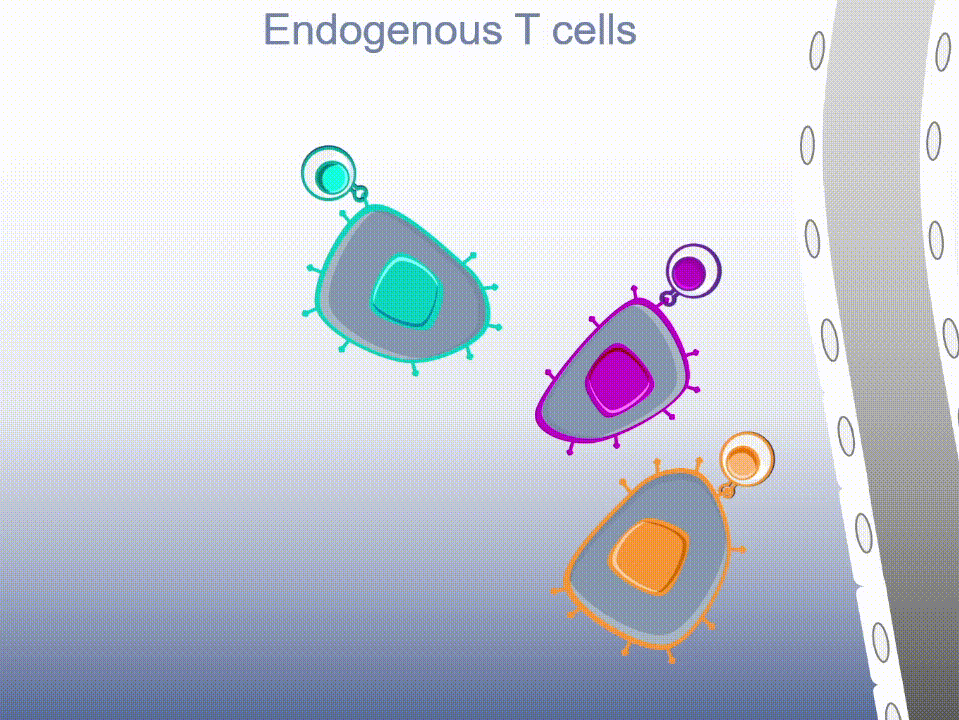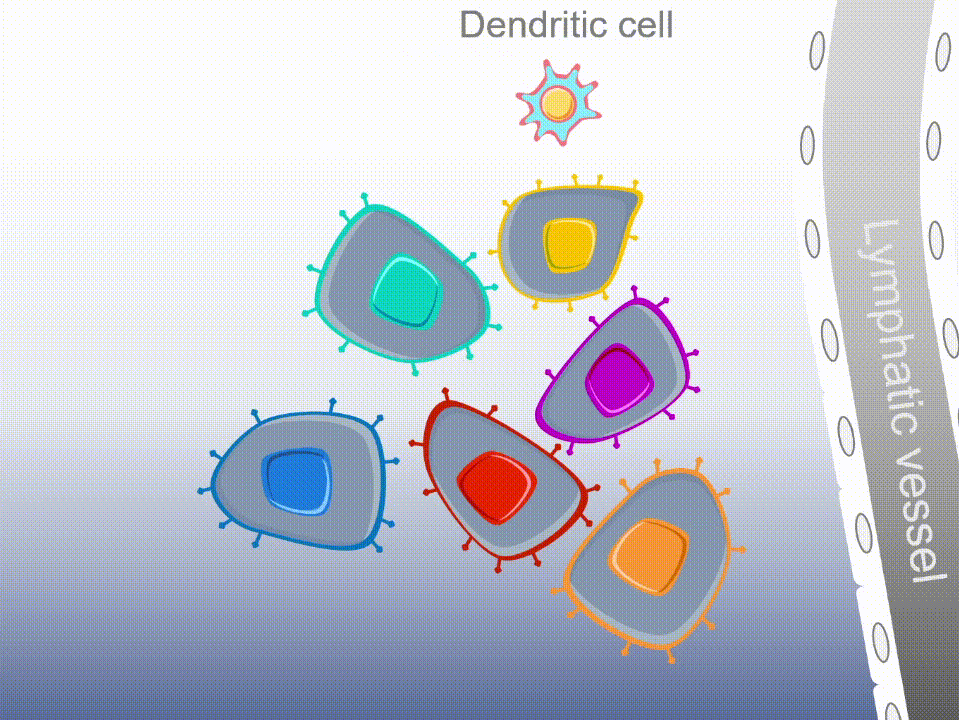
MultiTAA-specific therapies consistently demonstrate epitope spreading – inducing the patient’s own T cells to expand, potentially contributing to a lasting anti-tumor effect.
Clinical
Safety
MultiTAA-specific T cell therapies have been well-tolerated in clinical trials to date and may have reduced toxicities compared to current engineered CAR-T and T cell receptor-based therapies.
Natural
T Cells
MultiTAA-specific T cell therapies, which are based on the selective expansion of a patient’s natural T cells and are not genetically engineered, carry no mutagenesis risk.
Improved
Manufacturing
MultiTAA-specific T cells are significantly less expensive to manufacture than genetically engineered T cells. The cell manufacturing process is efficient and scalable.
Outpatient
Administration
Unlike other cell therapies which require pre-conditioning regimens and hospitalization, MultiTAA-specific T cell therapies are designed to be administered in an outpatient setting.